*All other brand or product names located in this site are Trademarks of their respective holders.
Montelukast Sodium Chewable Tablets
NDC number :
60505-3573-03
Material number :
59501
Active ingredient :
Montelukast Sodium
Bioequivalent to * :
SINGULAIR®
Therapeutic class (AHFS) :
Leukotriene Modifiers
Strength :
4MG
Pack size (form) :
30 TABLET;CHEWABLE (BOTTLE)
Dosage form :
Tablet, Chewable
Scored :
NO
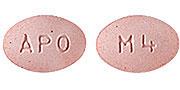
Color :
PINK
Shape :
OVAL, BICONVEX-SHAPED CHEWABLE TABLETS
Markings :
ENGRAVED "APO" ON ONE SIDE AND "M4" ON THE OTHER SIDE.
Route of administration :
Oral
Storage condition :
Controlled Room Temperature (68 - 77 degrees F)
Minimum order quantity :
12
Multiple order quantity :
12
Latex free :
Yes
Sugar free :
Yes
Dye free :
Yes
Alcohol free :
Yes
Preservative free :
Yes
Product resources :
Prescribing information (344 KB)

not to scale
Packaging information :
| Length | Width | Height | Weight | Units/Pack | |
|---|---|---|---|---|---|
| Unit | 1.8 in | 1.8 in | 3.3 in | 0.08 lb | N/A |
| Inner | 7.1 in | 5.3 in | 3.3 in | 0.93 lb | 12 |
| Case | 11.7 in | 8.1 in | 9.3 in | 6.06 lb | 72 |
