*All other brand or product names located in this site are Trademarks of their respective holders.
Risedronate Sodium Tablets
NDC number :
60505-3097-04
Material number :
54880
Active ingredient :
Risedronate Sodium
Bioequivalent to * :
ACTONEL®
Therapeutic class (AHFS) :
Bone Resorption Inhibitors
Strength :
150MG
Pack size (form) :
3 TABLET (BLISTER)
Dosage form :
Tablet, Film Coated
Scored :
NO
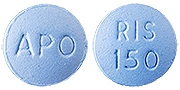
Color :
BLUE
Shape :
ROUND, BICONVEX FILM COATED TABLET
Markings :
ENGRAVED 'APO' ON ONE SIDE,'RIS' OVER '150' ON THE OTHER SIDE
Route of administration :
Oral
Storage condition :
Controlled Room Temperature (68 - 77 degrees F)
Minimum order quantity :
450
Multiple order quantity :
450
Bar code to unit-of-use :
Yes
Latex free :
Yes
Sugar free :
Yes
Dye free :
Yes
Alcohol free :
Yes
Preservative free :
Yes
Hazardous material :
No
Product resources :

 not to scale
not to scalePackaging information :
| Length | Width | Height | Weight | Units/Pack | |
|---|---|---|---|---|---|
| Unit | 2.3 in | 0.8 in | 3.8 in | 0.03 lb | N/A |
| Inner | 4.5 in | 3.8 in | 3.8 in | 0.3 lb | 10 |
| Case | 20.3 in | 13.6 in | 14.2 in | 13.95 lb | 450 |
